
Firmware & Embedded Systems
Mission-Critical Medical Device Firmware
From wearables to implantables
We develop safety-critical firmware for medical devices. Real-time performance, ultra-low power consumption, and bulletproof reliability for FDA Class II/III devices.
Medical Device Firmware Challenges
Embedded systems for healthcare require specialized expertise
Safety-Critical Requirements
Medical device firmware must meet strict safety standards with zero tolerance for errors or failures.
Power Efficiency
Wearable and implantable devices require efficient battery usage while maintaining continuous operation.
Real-Time Performance
Fast response times are required for critical medical measurements and interventions.
Security Vulnerabilities
Connected medical devices are targets for cyberattacks requiring robust security at the firmware and hardware level.
Wireless Connectivity
Implementing reliable Bluetooth, WiFi, and cellular while maintaining battery life and security.
Resource Constraints
Limited memory and processing power require highly optimized code and efficient algorithms.
Hardware Development Notice
Vintergatan does not offer hardware development services. We specialize in firmware development and can help you update existing proof-of-concept firmware, remediate legacy firmware issues, or develop production firmware from scratch in collaboration with your existing hardware engineering team.
Safety-Critical Development Process
Every line of code is written with patient safety in mind. Our firmware development follows IEC 62304 standards with comprehensive documentation and traceability.
Formal Verification
Mathematical proof of correctness for critical functions
Hardware Abstraction
Portable code across multiple microcontroller platforms
Power Optimization
Advanced sleep modes and energy harvesting support
AI at the Edge
TinyML models for on-device inference
Firmware Architecture
Application Layer
Medical Logic & Algorithms
Middleware
Communication • Security • Power
RTOS
FreeRTOS • Zephyr • SmartBASIC
Hardware Abstraction Layer
Drivers • Peripherals • DMA
Hardware
MCU • Sensors • Radios
Secure Update Architecture
Hardware Security Module
OTA Update Pipeline
Bulletproof Security & Updates
Medical devices require robust security. We build firmware that implements defense-in-depth with secure boot chains, and fail-safe update mechanisms. We also recommend using a hardware security module for additional security.
Hardware Security Modules
Dedicated secure elements for cryptographic operations and key storage
A/B Partition Updates
Dual-partition system ensures devices never brick during updates
Automatic Rollback
Self-healing firmware automatically reverts failed updates
Anti-Rollback Protection
Prevents downgrade attacks to vulnerable firmware versions
Multi-User Data Isolation
Cryptographically bonds medical data to individual users, preventing cross-contamination when devices are shared
Edge AI on Tiny Hardware
We've successfully deployed machine learning models on ultra-low-power MCUs. Real-time inference for medical diagnostics running for days on a coin cell battery.
On-Device Audio Processing
Real-time voice control, cough detection, and voice biomarkers
ECG Rhythm Classification
Detect arrhythmias, AFib, and abnormal heart patterns in real-time on 32-bit MCUs
Motion & Gait Analysis
Fall detection, Parkinson's tremor tracking, and rehabilitation monitoring using IMU data
Continuous Glucose Monitoring
Predictive glucose trend analysis and hypoglycemia warnings processed locally
TinyML Resource Constraints
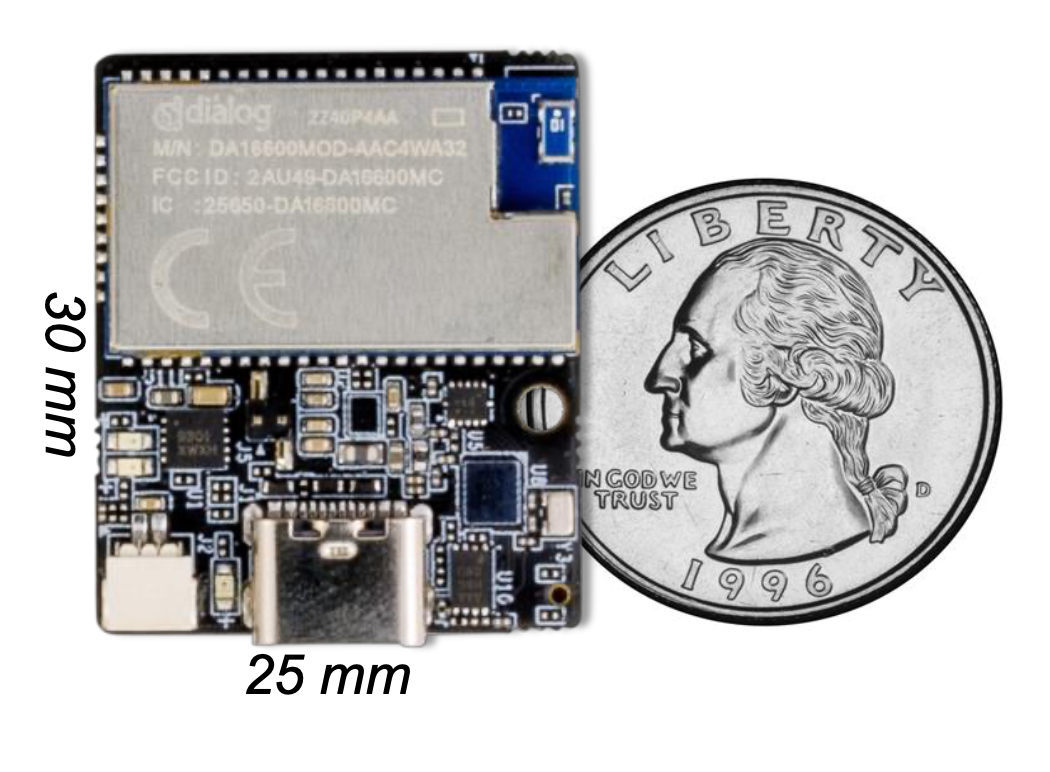
Modern Edge AI: Size of a Coin
Today's AI-capable microcontrollers are smaller than a fingernail yet powerful enough to run complex neural networks. With proper firmware optimization, these chips achieve remarkable efficiency.
The secret to battery life:
- Smart duty cycling can reduce average power by 99% in many cases
- Model parameter precision can be reduced all the way down to 1 bit for some models
Ultra-Low Power Duty Cycling for Edge AI
Purpose-built tools for FDA-compliant firmware
Our Custom Medical Device Testing Suite
We've developed our own comprehensive debugging and testing mobile application specifically for medical device firmware. This suite ensures complete FDA compliance, security validation, and performance optimization across iOS, Android, and embedded platforms.

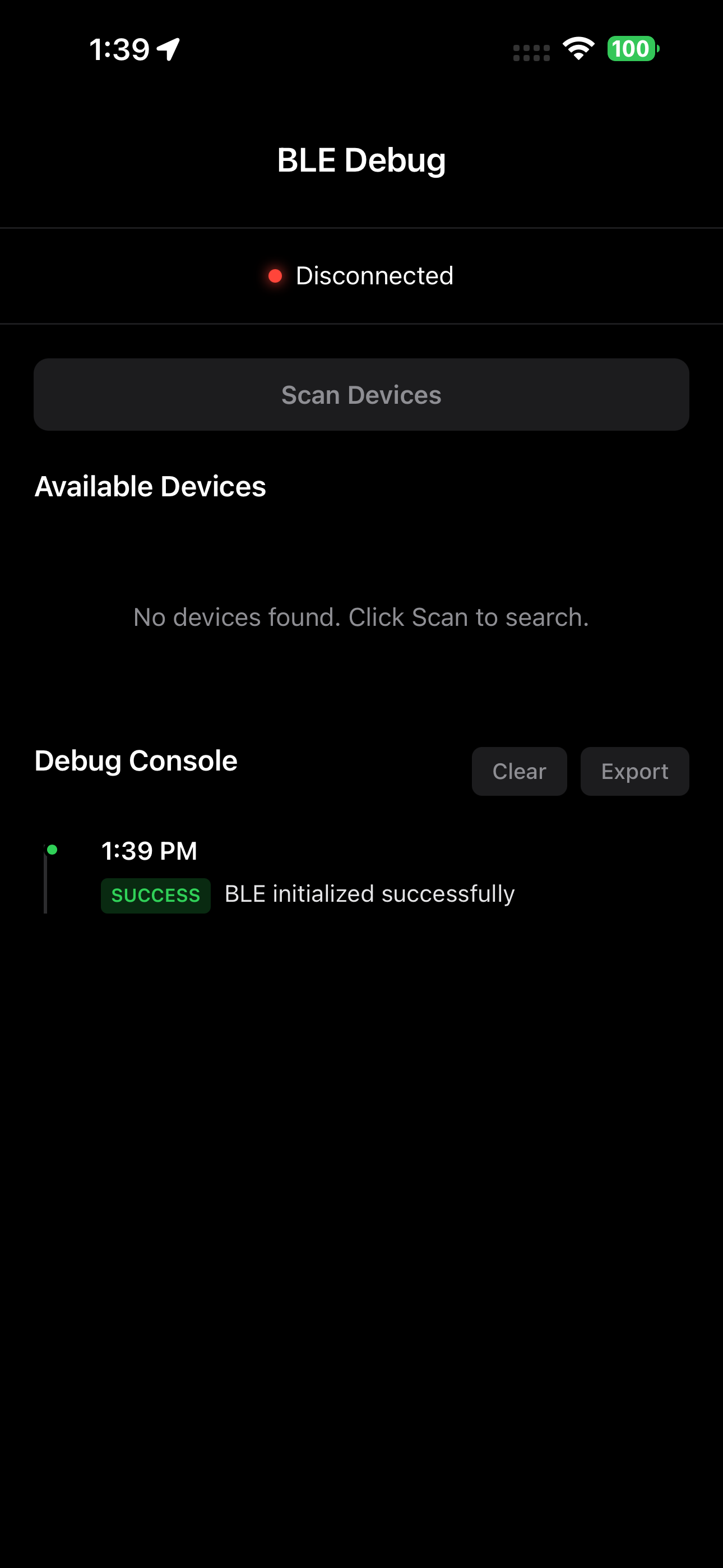
Real-time Debug Console
Monitor encrypted data streams and device connections
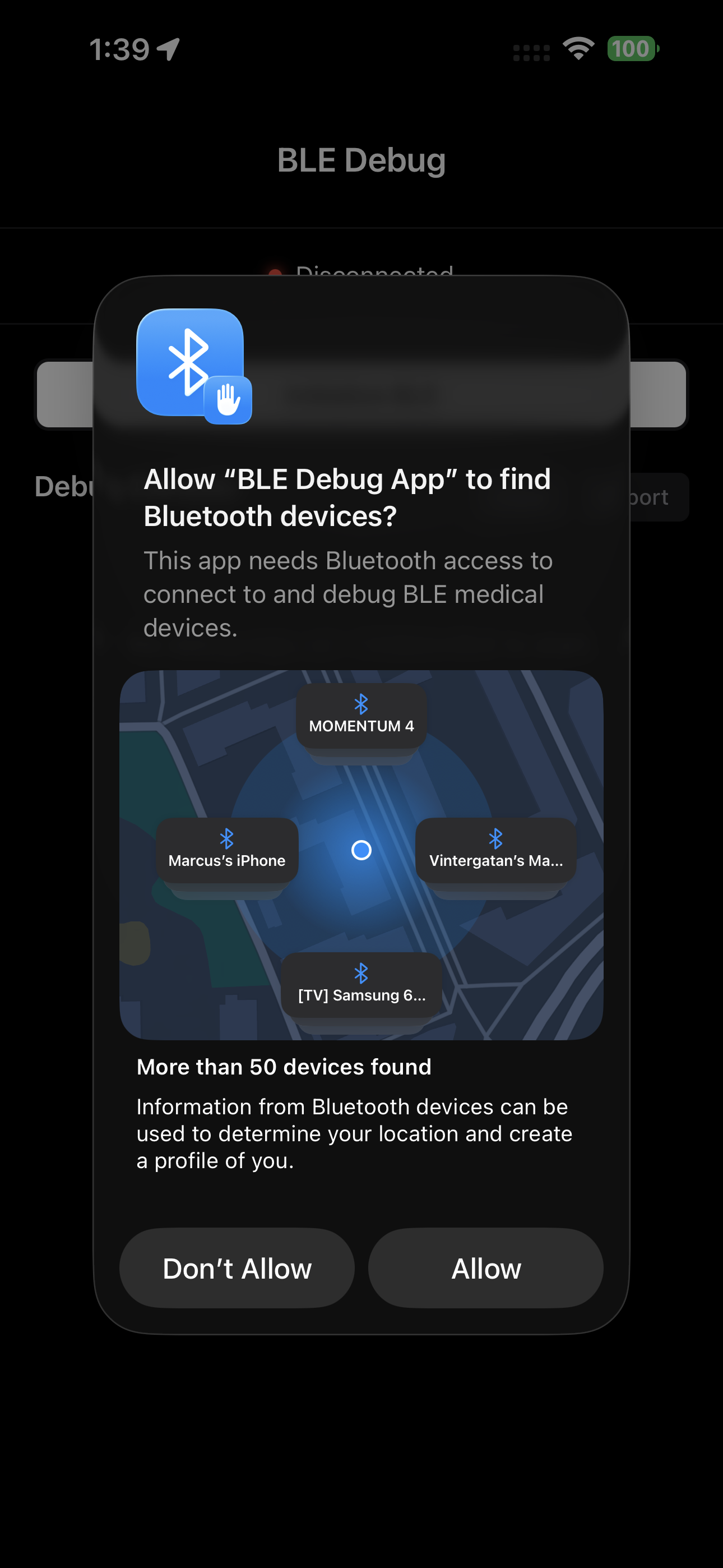
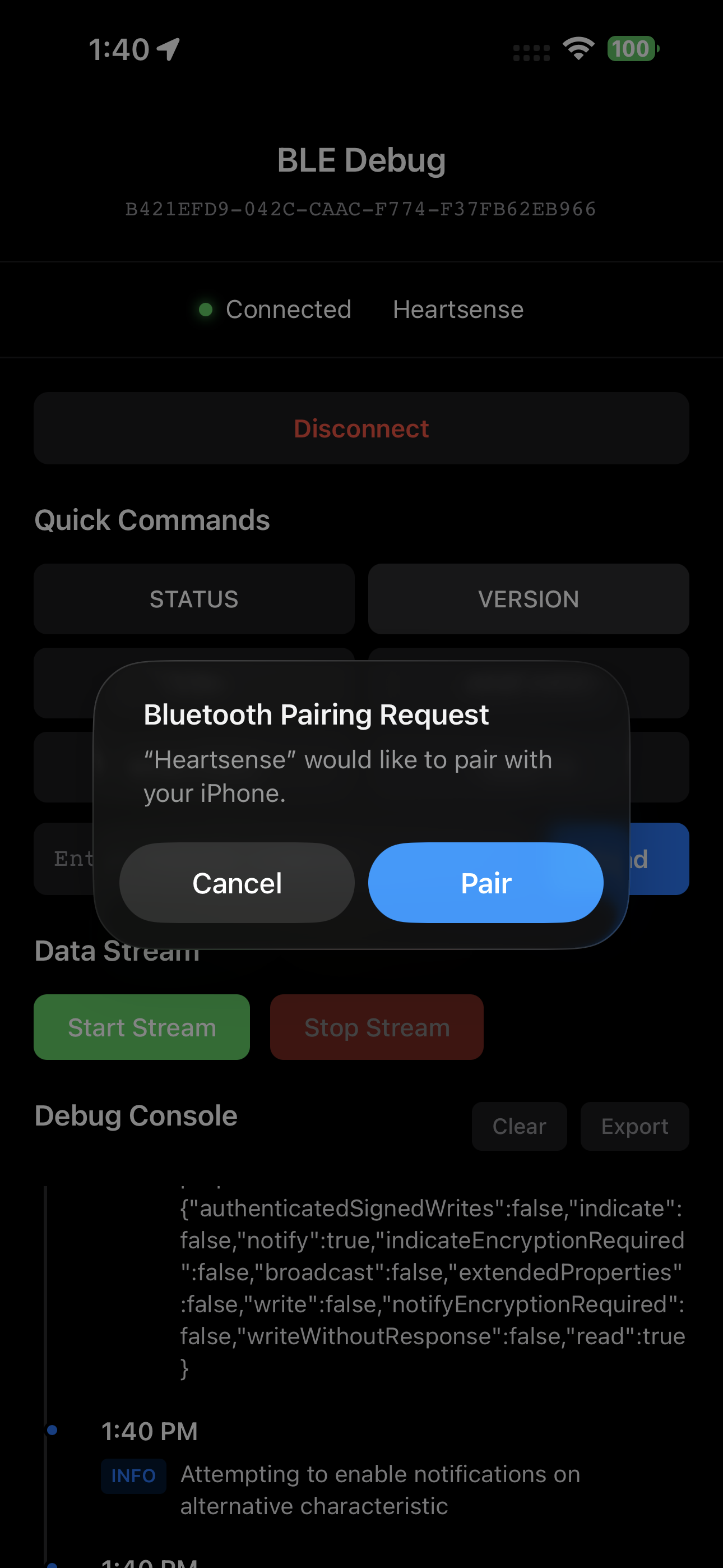
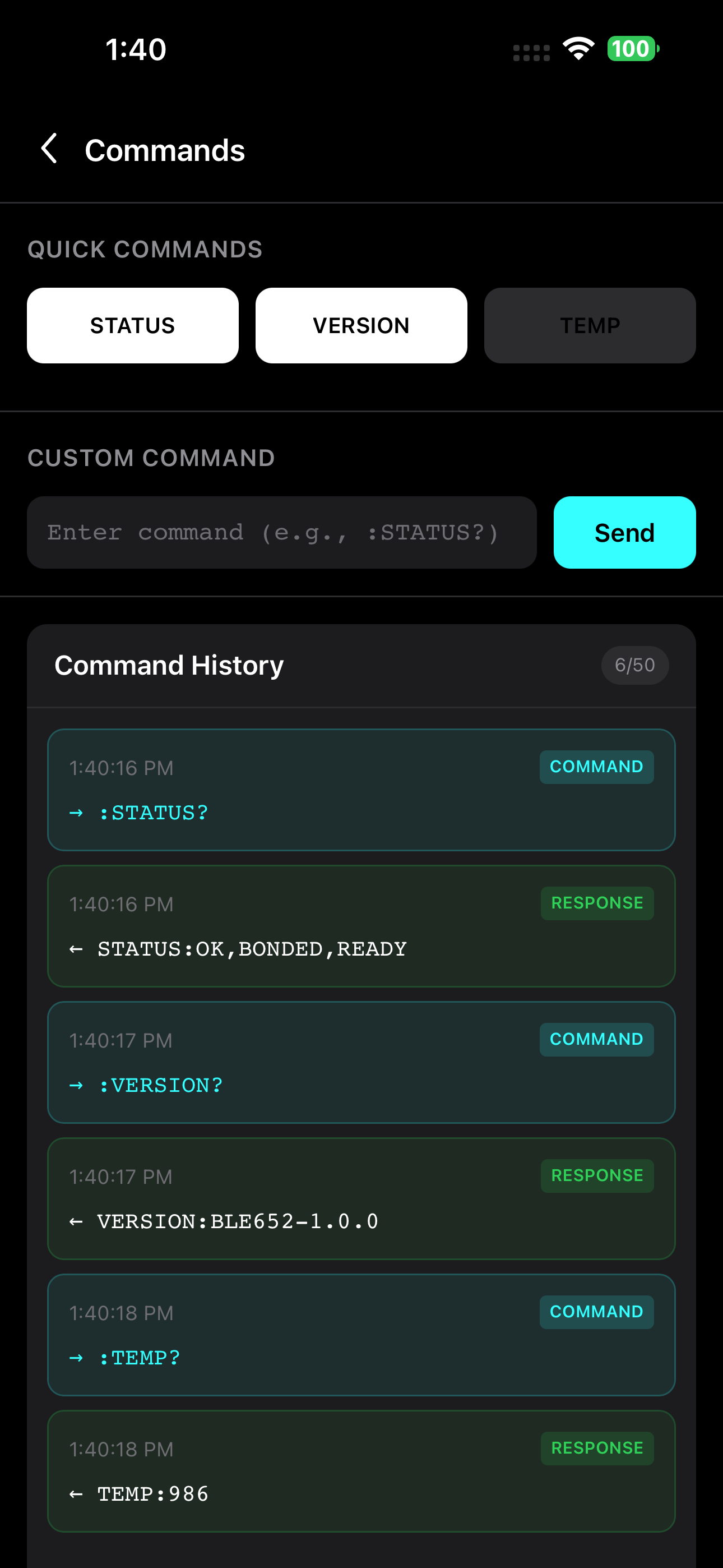
Secure Command Interface
Send validated commands with full audit logging
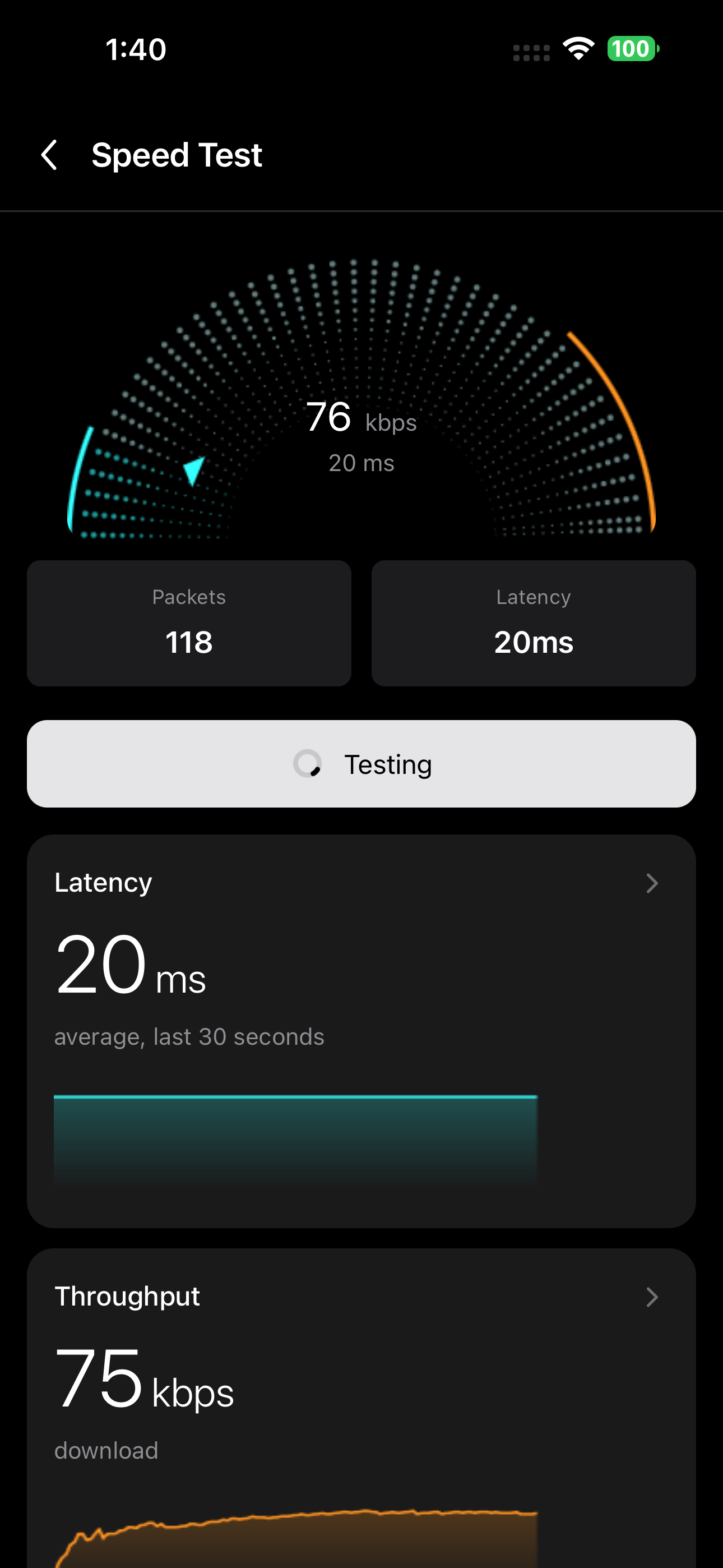
Performance Analytics
Real-time throughput and latency monitoring
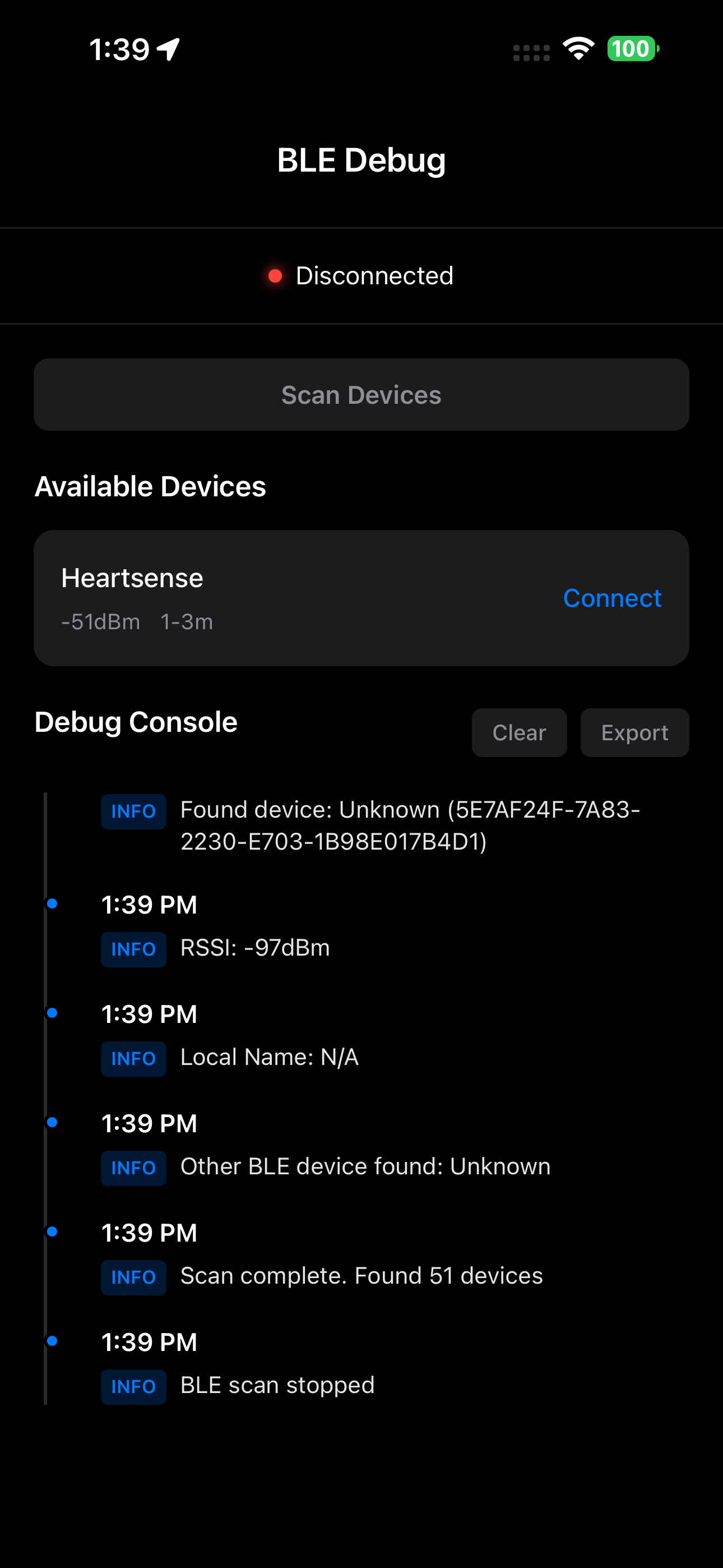
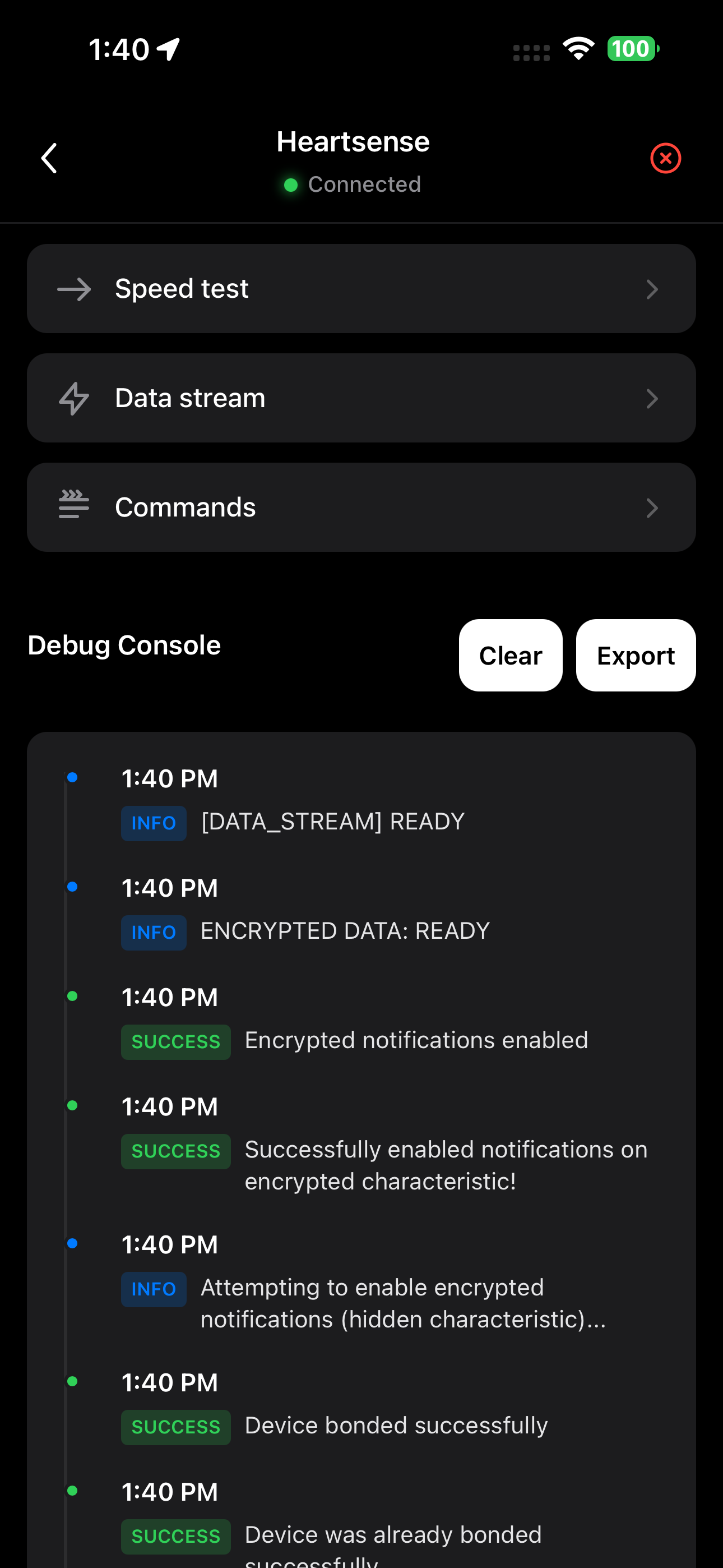
Device Connection Manager
Handle pairing, bonding, and encrypted connections
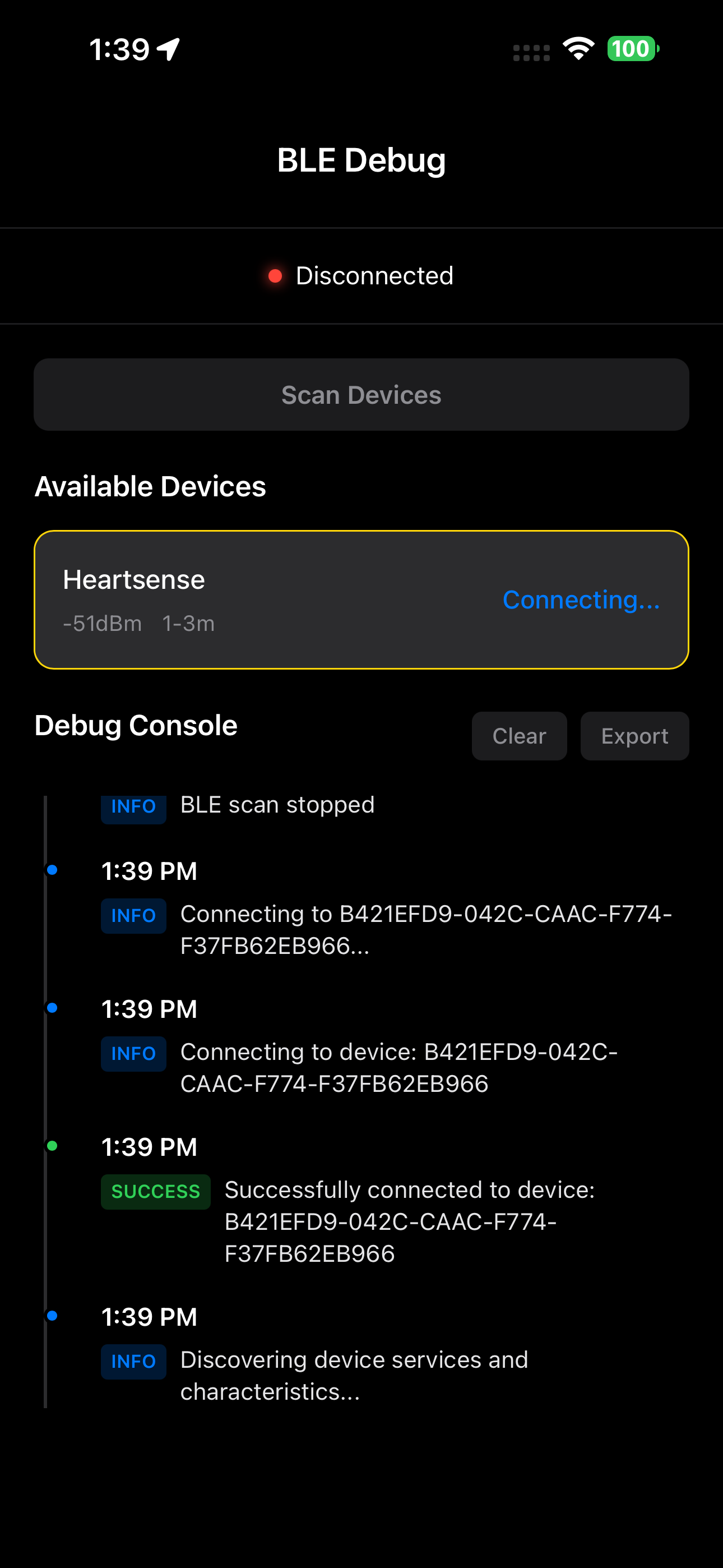
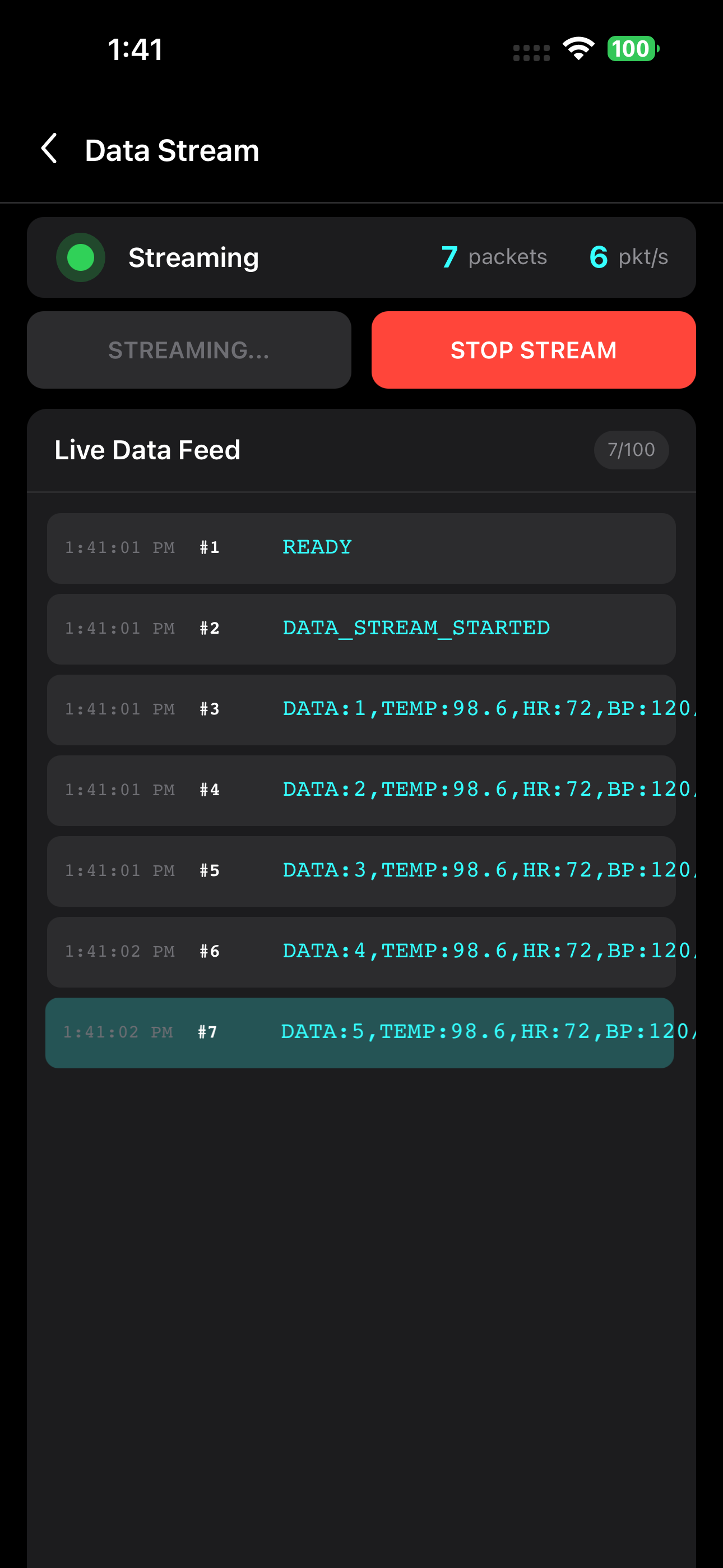
Live Data Streaming
Monitor continuous medical data with encryption
Protocol Validation
Test FDA-compliant communication protocols
Security Audit Tools
Verify encryption and authentication mechanisms
Battery & Power Analysis
Optimize power consumption for years of battery life
Advanced Diagnostics
Deep device inspection and troubleshooting
FDA Compliance Validation
Automated testing for regulatory requirements

Real-time Debug Console
Monitor encrypted data streams and device connections

Secure Command Interface
Send validated commands with full audit logging

Performance Analytics
Real-time throughput and latency monitoring

Device Connection Manager
Handle pairing, bonding, and encrypted connections

Live Data Streaming
Monitor continuous medical data with encryption

Protocol Validation
Test FDA-compliant communication protocols

Security Audit Tools
Verify encryption and authentication mechanisms

Battery & Power Analysis
Optimize power consumption for years of battery life

Advanced Diagnostics
Deep device inspection and troubleshooting

FDA Compliance Validation
Automated testing for regulatory requirements

Real-time Debug Console
Monitor encrypted data streams and device connections

Secure Command Interface
Send validated commands with full audit logging

Performance Analytics
Real-time throughput and latency monitoring

Device Connection Manager
Handle pairing, bonding, and encrypted connections

Live Data Streaming
Monitor continuous medical data with encryption

Protocol Validation
Test FDA-compliant communication protocols

Security Audit Tools
Verify encryption and authentication mechanisms

Battery & Power Analysis
Optimize power consumption for years of battery life

Advanced Diagnostics
Deep device inspection and troubleshooting

FDA Compliance Validation
Automated testing for regulatory requirements
Battle-Tested Development Tools
End-to-End Encrypted Connections
Our custom debugging app maintains FDA requirements for encrypted BLE and Bluetooth connections. We handle the complex differences between iOS, Android, and desktop implementations.
- AES-128/256 encryption for all data transfers
- Secure pairing with or without out-of-band (OOB) authentication
- Platform-specific security implementations
- Man-in-the-middle (MITM) attack prevention
Secure Command Protocol
Robust command validation prevents buffer overflows and system flooding. Every input is sanitized and validated before execution, reducing the attack surface.
- Input validation and sanitization
- Rate limiting to prevent flooding
- Command whitelisting and authentication
- Audit logging for forensic analysis
BLE Performance Optimization
Benchmark and optimize transfer speeds across different platforms. Our tools help maximize battery life by intelligently managing dual BLE/Bluetooth modules.
- Real-time throughput and latency monitoring
- Automatic Bluetooth deep sleep when idle
- Dynamic connection parameter optimization
- Power consumption profiling
FDA-Mandated Validation & Testing
Comprehensive testing and validation isn't optional—it's a legal requirement. Our debugging tools ensure complete FDA compliance for medical device firmware submissions.
- Software validation plan defining scope, approach, and acceptance criteria
- Complete V&V documentation aligned with regulatory requirements
- Test results demonstrating safety and effectiveness
- Quality system regulation compliance (21 CFR Part 820)
- Predetermined requirements verification
Embedded Technology Stack
Low-level languages and real-time operating systems
Medical Device Development Process
From concept to certified firmware
Click any card below to explore details
System Requirements & Safety Analysis
Define hardware specifications, safety requirements, and regulatory constraints for the medical device.
Hardware & Firmware Prototype
Develop proof-of-concept firmware on development boards to validate technical approach.
Production Firmware Development
Implement production-grade firmware with safety features, error handling, and optimization.
Verification & Validation
Comprehensive testing including unit tests, integration tests, and hardware-in-the-loop testing.
Regulatory Documentation
Prepare comprehensive documentation aligned with FDA guidance and other regulatory standards.
Medical Device Standards
Firmware meeting the highest safety and quality standards
IEC 60601
Medical electrical equipment safety
IEC 62304
Following medical device software lifecycle
ISO 14971
Risk management for medical devices
FDA Class II/III
Aligned with FDA AI/ML guidance
CE Mark
Aligned with European regulatory standards
UL 2900
Cybersecurity for connected devices
IEC 60601-1-2
Electromagnetic compatibility
ISO 10993
Biocompatibility
All systems undergo rigorous security audits and penetration testing
Ready to Build Your Medical Device?
Partner with experts who understand medical device requirements
Other Services
Explore our complete range of healthcare technology solutions
Healthcare AI Solutions
AI systems that reduce documentation burden and improve diagnostic accuracy. From ambient clinical documentation to predictive analytics. Built to FDA/CE standards with complete technical documentation.
Learn moreMedical Device Platforms
End-to-end platforms connecting devices to clinical insights. Real-time data processing, device management, and analytics at scale. Designed for ISO 13485 and FDA compliance from day one.
Learn moreMobile & Web Applications
Patient apps people actually use and clinicians trust. Telemedicine platforms, wellness trackers, and clinical workflows. Beautiful design with HIPAA compliance built in.
Learn moreEnterprise IT Solutions
Transform fragmented healthcare IT across multi-site health systems. Legacy modernization, EHR integration, and cloud migration. Scale to millions of patients with 99.99% uptime.
Learn moreInternal Research Tools
Custom tools that automate workflows and deliver real-time insights. Clinical trial management, operational dashboards, and research platforms. Built specifically for your team's processes.
Learn more